how to draw molecular orbital diagram of no
Determine point group of molecule if linear use D2h and C2v instead of Dh or Cv 2. This article explains how to create molecular orbital diagrams in L a T e X by means of the package MOdiagramFor information about the more traditional molecular structure diagrams see our documentation about chemistry formulae.
Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds.
. 8 - Drawing Molecular Orbital Diagrams. Co molecule has 10 valence electronsfour from carbon atom 2s²2p² and six from oxygen atom 2s²2p⁴according to molecular orbital diagram molecular orbital configuration is given as σ2s² σ2s² πx² πy² σz² πx⁰ πy⁰ σz⁰ Label. Sp Hybrid Orbitals in BeH2 1.
There are a total of 6 electrons to add to the molecular orbital diagram 3 from boron and 1 from each hydrogen atom. It is a linear molecule. Since more than one atom is involved we refer to these orbitals as molecular orbitals.
Related
These are UVVisible Infra-red IR and Nuclear Magnetic Resonance NMR spectroscopies. Molecular orbital diagram of N 2 is shown below. Introduction to Molecular Spectroscopy.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals. To obtain the bond order look at the molecular orbitals formed and decide whether they are bonding or antibonding.
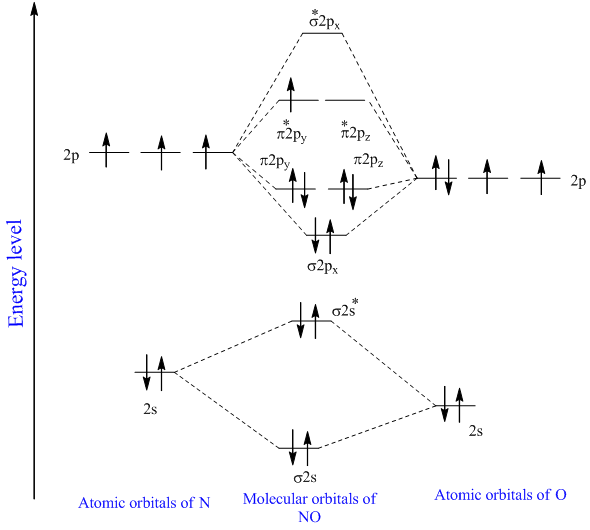
Molecular Orbital Diagram of NO. Considers bonds as localized between one pair of atoms. The applications of the MO theory extend beyond the limitations of the Valence Shell Electron Pair Repulsion VSEPR model and the Valence Bond theory.
So no electrons should be in that orbital and then finally once you have everything drawn fill the molecular orbitals according to the rules of electron configuration which would be Aufbau principle you have to build up Pauli exclusion you can only put two electrons in each orbital and hunds rule you have to fill or equal energy orbitals. Creates bonds from overlap of atomic orbitals s p d and hybrid orbitals sp sp2 sp3 combines atomic orbitals to form molecular orbitals σ σ π π forms σ or π bonds. Bond order bonding electrons - antibonding electrons 2.
Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules. Orbitals represented by are antibonding orbitals and the orbitals without are bonding orbitals. Compare the bond order to that seen in the Lewis structure remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital.
The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s antibonding molecular orbital just like the 1s and 1s orbitals formed from the 1s atomic orbitals.
If non-linear y axes of outer atoms point to central atom3. Depending on if it is a homonuclear case where the bonding atoms are the same or a heteronuclear case where the bonding atoms are. When considering bonding between two atoms first construct the relevant molecular orbitals then fill them with the available electrons starting with the lowest energy molecular orbital.
See Resources for a diagram showing the filling order. Find the characters of the reducible representationfor the combination of. Next well see that symmetry will help us treat larger.
Bond order can be calculated by the formula. Molecular orbital diagram as a non-bonding molecular orbital. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.
Because the electronegativity of the two atoms are unequal the molecular orbital diagram will no longer be symmetric. The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. Molecular Orbitals of the Second Energy Level.
Construct a qualitative molecular orbital diagram for chlorine Cl 2. Molecular Orbital Theory. In this case were using the standard one.
Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. Assign x y z coordinates z axis is principal axis.
How to Build Molecular Orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms the 2p z. The molecular orbital MO theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms.
Each orbital can accommodate a maximum of two electrons but. And this should make sense because NO is isoelectronic with CO which has a bond order of 3. I was just wondering if the same applied for molecules with a.
The content is presented using short focussed and. When two or more atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. With one additional electron in an antibonding.
Considers electrons delocalized throughout the entire molecule. The purpose of MO theory is to fill in the gap for some. Molecular Orbitals for Larger Molecules 1.
Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. Instead the more electronegative element is drawn lower in. Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom.
BO 1 2 bonding e antibonding e 1 2 2 2 2 2 2 1 25. Next article molecular orbital diagram of no. In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative.
This picture shows the molecular orbital diagram of N 2.
Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com
Delocalized Bonding And Molecular Orbitals
What Is The Molecular Orbital Diagram For No Quora
Solved Chapter 5 Problem 7p Solution Inorganic Chemistry 5th Edition Chegg Com
Draw The Diagrams For No 2 No 2 And No 2 The Homo Of No 2 Shows It Is Somewhat Anti Bonding Would You Expect The Nonbonding Electron Pairs On Nitrogen Or Oxygen To Be More Reactive
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community
File Nitric Oxide Mo Diagram Svg Wikimedia Commons
What Is The Molecular Orbital Diagram For No Quora
